Research News
Dr. Erquan Zhang's group reported a real-time monitoring system for the brain clock genes
In collaborating with the NIBS Imaging Core Facility, scientists in Dr. Erquan Zhang's laboratory have invented a GFP-based, long-term real-time monitoring system to investigate the dynamic expressions of brain clock genes in live animals. On April 2, 2018, this study was published online in PNAS entitled " Long-term in vivo recording of circadian rhythms in brains of freely moving mice ". In addition, a video-based, detailed procedure paper has been accepted and will soon be published in a methodology journal, J Vis Exp.
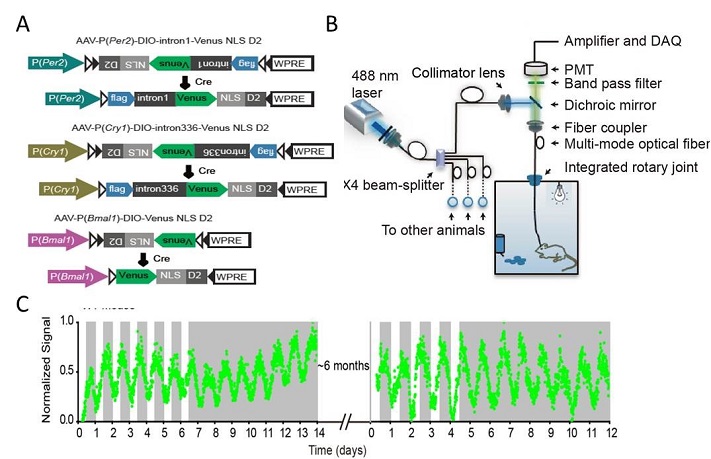
A new system for
long-term recording the brain clock genes in vivo. (A) rAAV constructs design.
(B) the instrumental set-up. (C) an example for long-term Cry1 reporter recording in light/dark (LD) or constant dark (DD)
conditions.
Dissecting the transcriptional regulation is critically important for understanding various biological aspects, since gene transcription is tightly engaged in controlling our behavior and physiology. Conventional approaches, including qPCR, in situ hybridization, western blotting, and luciferase reporter assays, are widely used but indeed not well suitable to be applied for circadian clock studies. The disadvantages of these methods comprise, but not limited to, difficulty in measuring under in vivo conditions, not real-time, labor-intensive and expensive, less reproducible, etc. A few years ago, we decided to develop a fluorescence reporter-based monitoring system to measure the clock gene transcription in vivo. Mr. Long Mei, a graduate student in the Zhang lab and the lead author of both papers, created a serial constructs harboring the cis-elements of Cry1 and Per2 core clock genes fused with a destabilized Venus reporter, and successfully recaptured the expression patterns of corresponding genes in cell cultures. Subsequently, he generated adeno-associate viruses (rAAVs) and delivered the reporters into targeted neurons in live mice. Meanwhile, Dr. Zhan's group of the Imaging Core have developed a reliable high-throughput, opti-fiber based, recording instrument. Together, the research team achieved the goal for long-term real-time monitoring clock genes' expression in the brains of freely moving mice.
Using this system, scientists was first-time ever able to continuously record the oscillating expression of the brain Cry1 gene for more than 6 months in a single mouse. The research team also investigated how the circadian genes in VIP neurons (located in the clock master SCN) respond to the jet-lag treatment. Combining with the Cre-loxP system, they demonstrated that the phase advance of 8 h would result in dramatic changes of Per2 and Cry1 expressions, and they took more than 6 days to completely restore to normal levels.
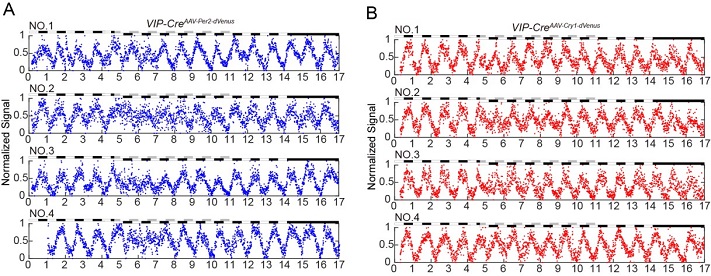
The Nobel Prize of Physiology and Medicine in 2017 was awarded to three scientists for their cloning of the first clock gene, period. Circadian research relies on long-term monitoring methods, which usually take days to weeks, or even months, for the recording and sampling. Comparing to conventional methods, this new technique made significant advances in several fronts: 1) suitable for long-term continuous recording (for months); 2) high temporal resolution (10 min interval for each detection); 3) live imaging from freely moving mice; 4) capable for manipulating under light conditions, which are critical for circadian studies. Most importantly, this technique, once properly modified, can be applied to detect the gene expressions beyond the scope of circadian research.
Long Mei, a PTN program PhD student in Dr. Erquan Zhang’s lab, is the first author of this paper. Dr. Erquan Zhang is the corresponding author, and Dr. Cheng Zhan is the co-corresponding author. Other contributing authors include Yanyan Fan of the NIBS Imaging Core; Dr. Xiaohua Lv from Huazhong University of Science and Technology; and Dr. David K. Welsh from University of California San Diego. This study was supported by grants from NNSFC, MOST 973 program, and Beijing Municipal Government. All works were completed in NIBS.



