Research News
A new genome protection mechanism discovered by Dr. Li-Lin Du’s laboratory
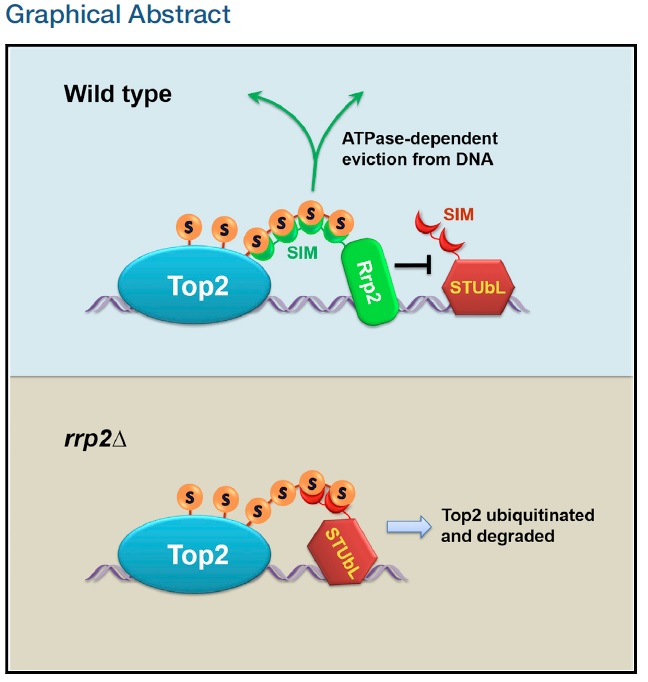
On May 25, 2017, Dr. Li-Lin Du 's lab published in Molecular Cell a paper titled "SUMO-Targeted DNA Translocase Rrp2 Protects the Genome from Top2-Induced DNA Damage". The authors show that the fission yeast protein Rrp2 safeguards the genome from damage induced by chemicals poisoning DNA topoisomerase II (Top2), such as the anti-cancer drug etoposide.
The action of DNA topoisomerase II (Top2) creates transient DNA breaks that are normally concealed inside Top2-DNA covalent complexes. Top2 poisons, including ubiquitously present natural compounds and clinically used anti-cancer drugs, trap Top2-DNA complexes. In this paper, the authors show that cells actively prevent Top2 degradation to avoid the exposure of concealed DNA breaks. A genome-wide screen revealed that fission yeast cells lacking Rrp2, an Snf2-family DNA translocase, are strongly sensitive to Top2 poisons. Loss of Rrp2 enhances SUMOylation-dependent ubiquitination and degradation of Top2, which in turn increases DNA damage at sites where Top2-DNA complexes are trapped. Rrp2 possesses SUMO-binding ability and prevents excessive Top2 degradation by competing against the SUMO-targeted ubiquitin ligase (STUbL) for SUMO chain binding and by displacing SUMOylated Top2 from DNA. The budding yeast homolog of Rrp2, Uls1, plays a similar role, indicating that this genome protection mechanism is widely employed, a finding with implications for cancer treatment.
Dr. Yi Wei from Dr. Li-Lin Du’s lab is the first author of this article. Other contributors include Li-Xue Diao, Dr. Hai-Tao Wang, Fang Suo from Dr. Li-Lin Du’s lab, and Dr. Shan Lu and Dr. Meng-Qiu Dong from Dr. Meng-Qiu Dong’s lab. Dr. Li-Lin Du is the corresponding author of this article. This project was supported by the Chinese Ministry of Science and Technology, the Beijing municipal government, and the National Natural Science Foundation of China.
Web address of the paper:http://www.cell.com/molecular-cell/fulltext/S1097-2765(17)30273-3