Research News
A study from Dr. Erquan Zhang's laboratory reveals that hypoxia signaling and the circadian clock crosstalk at the genome level.
In the
current issue of Cell Metabolism available
from Jan 10, 2017, Dr. Erquan Zhang's lab at NIBS published a featured research
article entitled “Reciprocal Regulation between the Circadian Clock and Hypoxia
Signaling at the Genome Level in Mammals”. In this article, they described a
long-awaited and detailed mechanism of the reciprocal regulation between the
circadian clock and hypoxia signaling in higher animals such as rodents and
humans.
It
is known that circadian regulation plays an important role in maintaining
metabolic and physiological homeostasis. The mammalian clock consists of a
"core" transcriptional feedback loop with CLOCK/BMAL1 as positive
factors and PERIOD/CRYPTOCHROME (or PER/CRY) as negative regulators. These
components work in concert to generate a rhythm of roughly 24h duration. It has
also been established that multiple "stabilizing" feedback loops are
associated with the core loop; these function in structuring a hierarchical,
multi-layered regulatory network. In 2009, Dr. Zhang’s genome-wide
small-interfering (si)RNA screen established that many other cellular pathways,
including cell-cycle signaling, both impinge on and are affected by the
circadian clock. Thus, the mammalian clock is intertwined with various
signaling networks and profoundly influences multiple aspects of our behavior
and physiology.
Hypoxia
signaling is also known to regulate both physiology and pathology in mammals.
The physiological responses that occur during hypoxia include angiogenesis,
metastasis, and erythropoietin (EPO) production, and the pathological events are
involved in stroke and heart-attack. The assumption that the clock and hypoxia
signaling may crosstalk can be traced back to the molecular cloning era, when the
key hypoxia signaling molecules HIF1A/ARNT were found to be structurally
similar, respectively, to the circadian components CLOCK/BMAL1 (Hogenesch et
al., PNAS, 1998). However, the
subsequent attempts to link these two pathways together all failed, at least
partially due to the side-effects and toxicity of conventional hypoxia inducers
like Ni2+ and Co2+. To investigate the possible impact of
the clock on hypoxia-responsive signaling in
vivo, the Zhang research team examined the induction of known
hypoxia-regulated genes in response to a fixed stimulation during a
time-course. Intraperitoneal injection of dimethyloxalylglycine (DMOG), a novel
low-toxicity compound used to mimic hypoxia, induced the expression of
Hif1a-target genes, including Glut1, Vegfa, Epo, and Egln1-3, in a
time-dependent manner. Expression of these genes was highly induced at zeitgeber time (ZT) 6-9 in the livers of
DMOG-treated mice, which coincides with the peak expression of E-box genes. Similarly,
a stronger induction of EPO by the clinical trial III drug FG-4592 (an EGLNs-specific
inhibitor that stabilizes HIF1A and
activates hypoxia signaling) was observed in the kidney. Thus, a so-called
"circadian gating" phenomenon occurs widely for hypoxia signaling in vivo.
To
further dissect the molecular mechanism underlying this phenotype, the Zhang
group used approaches from molecular and cellular biology, bioinformatics, and
functional genomics such as ChIP-seq analysis, and unambiguously demonstrated that
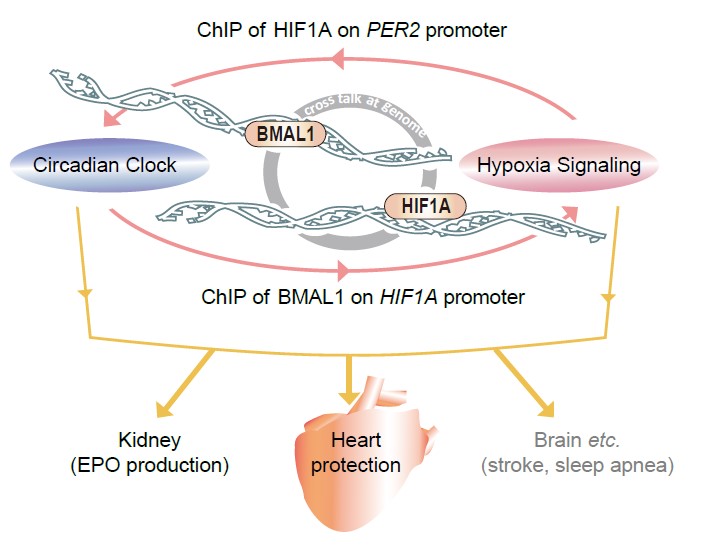
the clock-hypoxia crosstalk exists with at least two-layers: (A) at the
chromatin level, BMAL1 and HIF1A synergistically activate their co-occupant
targets, including genes involved in the clock and hypoxia regulations; (B) at
the gene transcription level, BMAL1 binds to and activates the HIF1A gene; and HIF1A conversely
regulates PER2 expression, thereby
slowing down and crippling the clock. This mutual regulatory mechanism is further
supported by two simultaneously-published short articles in the same journal (Peek
et al., and Adamovich et al., 2017).

Last,
but not the least, a heart-attack model was established in mice by using
coronary artery ligation; this model was used to show that that induced heart
damage varies in severity according to circadian patterns. Moreover, mutant
mice with a disrupted clock (Per1/Per2 double knockout) displayed significantly more severe myocardial injury than did
control mice. This could be interpreted as owing to a stronger hypoxic response
in mutant mice, especially the induction of the proapoptotic gene Bnip3, ultimately resulting in more
severe cell death. Thus, normal function of the clock may be a crucial
protective factor during heart-attacks.
The PhD student Yaling Wu from Beijing Normal University and the PTN graduate student Dingbin Tang in the Zhang lab are the two co-first authors. Other contributing authors include Na Liu, Wei Xiong, Yang Li, Zhixiong Ma, and Haijiao Zhao from the Zhang lab, and Dr. Xiangbing Qi, Mr. Peihao Chen, and Mr. Huanwei Huang from the NIBS core facilities. Dr. Erquan Zhang is the corresponding author. This research was supported by a grant of the 973 Program (2012CB837702) from the M.O.S.T. of China, the Beijing Municipal Government, and the Chinese "Recruitment Program of Global Youth Experts" (to Dr. Zhang), and was carried out at the National Institute of Biological Sciences, Beijing. The cover image was designed by Mrs. Mengli Shi at NIBS.



