Research News
Studies from Dr. TaoWang's laboratory demonstrate that the phosphorylation modification of PINK1 and Parkin play key roles in mitochondrial quality control
Publication Date:2016/12/07
Dec. 1st, 2016-Studies from Dr. Tao Wang's laboratory revealed that phosphorylation modifications on Drosophila PINK1 S346 residue and Parkin S94 residue are both required for activation of the PINK1/Parkin pathway. The work entitled “PINK1-dependent Phosphorylation of PINK1 and Parkin Is Essential for Mitochondrial Quality Control” is published online in the journal Cell Death & Disease(7(12):e2501. doi: 10.1038/cddis.2016.396).
Mitochondria play a fundamental role in eukaryotic metabolic processes by generating adenosine triphosphate (ATP) as a cellular energy source. Dysfunctional mitochondria deprive cells of energy, produce toxic reactive oxygen species (ROS) and other pro-death mediators to initiate cell death. The mitochondrial quality control pathways that evolved to maintain the integrity of mitochondria therefore play key roles in the normal function of cells. The pathogenesis of a large number of inherited diseases in humans, including Parkinson’s disease, has been linked to mitochondrial dysfunction. Previous research has reported that Parkinson’s disease genes pink1 and parkin participate in mitochondria quality control by promoting the selective removal of mutant mitochondria, but how Parkin selectively labels dysfunctional mitochondria for their lysosome degradation remains further investigation.
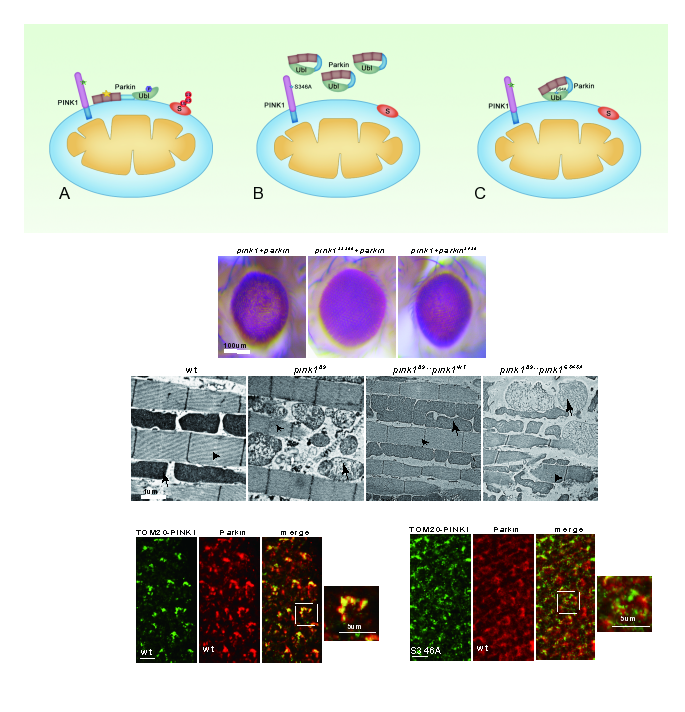
In the current study, Dr. Tao Wang and his team established an in vivo PINK1/Parkin-induced photoreceptor neuron degeneration model in Drosophila with the aim of dissecting the PINK1/Parkin pathway in detail. Through Phos-tag gel electrophoresis technology, the authors found that Drosophila PINK1 undergoes auto-phosphorylation modification, and by using LC-MS/MS analysis, the authors identified Serine 346 as the sole autophosphorylation site of Drosophila PINK1. Overexpression of mito-targeted PINK1 and Parkin in adult fly eye caused the degeneration of photoreceptor neuron,and substitution of Serine 346 to Alanine completely abolished the PINK1 autophosphorylation, thus fully inhibited PINK1/Parkin mediated cell death phenotype. As the substrate of PINK1 kinase, phosphorylation of Parkin by PINK1 is also important for mitochondria quality control, and disruption of PINK1 dependent Parkin phosphorylation largely alleviated the PINK1/Parkin induced neurodegeneration. This study further revealed that phosphorylation of PINK1 is required for both mitochondrial recruitment of Parkin and induction of its kinase activity toward Parkin. In contrast, phosphorylation of Parkin by PINK1 is dispensable for its translocation, but required for its activation. Moreover, substitution with autophosphorylation-deficient PINK1 failed to rescue pink1 null mutant phenotypes, indicating that autophosphorylation of PINK1 on Serine 346 plays a key role in its function in mitochondrial quality control in vivo.In conclusion, this study suggested a model of recruitment of Parkin by PINK1 kinase, and established a powerful genetic system in Drosophila to study the pathogenic mechanisms of Parkinson's diseases.
Mitochondria play a fundamental role in eukaryotic metabolic processes by generating adenosine triphosphate (ATP) as a cellular energy source. Dysfunctional mitochondria deprive cells of energy, produce toxic reactive oxygen species (ROS) and other pro-death mediators to initiate cell death. The mitochondrial quality control pathways that evolved to maintain the integrity of mitochondria therefore play key roles in the normal function of cells. The pathogenesis of a large number of inherited diseases in humans, including Parkinson’s disease, has been linked to mitochondrial dysfunction. Previous research has reported that Parkinson’s disease genes pink1 and parkin participate in mitochondria quality control by promoting the selective removal of mutant mitochondria, but how Parkin selectively labels dysfunctional mitochondria for their lysosome degradation remains further investigation.
In the current study, Dr. Tao Wang and his team established an in vivo PINK1/Parkin-induced photoreceptor neuron degeneration model in Drosophila with the aim of dissecting the PINK1/Parkin pathway in detail. Through Phos-tag gel electrophoresis technology, the authors found that Drosophila PINK1 undergoes auto-phosphorylation modification, and by using LC-MS/MS analysis, the authors identified Serine 346 as the sole autophosphorylation site of Drosophila PINK1. Overexpression of mito-targeted PINK1 and Parkin in adult fly eye caused the degeneration of photoreceptor neuron,and substitution of Serine 346 to Alanine completely abolished the PINK1 autophosphorylation, thus fully inhibited PINK1/Parkin mediated cell death phenotype. As the substrate of PINK1 kinase, phosphorylation of Parkin by PINK1 is also important for mitochondria quality control, and disruption of PINK1 dependent Parkin phosphorylation largely alleviated the PINK1/Parkin induced neurodegeneration. This study further revealed that phosphorylation of PINK1 is required for both mitochondrial recruitment of Parkin and induction of its kinase activity toward Parkin. In contrast, phosphorylation of Parkin by PINK1 is dispensable for its translocation, but required for its activation. Moreover, substitution with autophosphorylation-deficient PINK1 failed to rescue pink1 null mutant phenotypes, indicating that autophosphorylation of PINK1 on Serine 346 plays a key role in its function in mitochondrial quality control in vivo.In conclusion, this study suggested a model of recruitment of Parkin by PINK1 kinase, and established a powerful genetic system in Drosophila to study the pathogenic mechanisms of Parkinson's diseases.
Na Zhuang, a graduate student jointly trained by NIBS, TsingHua University and Peking University is the first author of this article. Other contributors include Lin Li, and Dr. She Chen from NIBS. Dr. Tao Wang is the corresponding author. The study was supported by the Chinese Ministry of Science and Technology 973 Grants, and was conducted at the National Institute of Biological Sciences, Beijing.