Research News
Aug. 1, 2016 -Studies from Dr. Feng Shao’s laboratory reveal that phosphorylation modification on specific serine residues in Pyrin and dynamics of microtubule structure are both important for activation of the Pyrin inflammasome in innate immunity. The w
Aug. 1, 2016 -Studies from Dr. Feng Shao’s laboratory reveal that phosphorylation modification on specific serine residues in Pyrin and dynamics of microtubule structure are both important for activation of the Pyrin inflammasome in innate immunity. The work entitled “Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation” is published online in the journal Proc. Natl. Acad. Sci. USA.
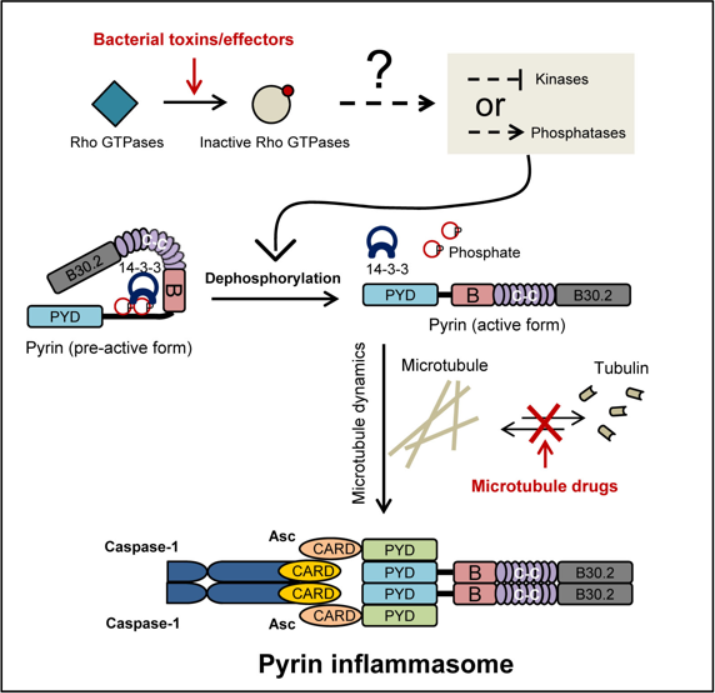
Cytosolic innate immunity is critical for limiting and combating microbial infections. Inflammatory caspases including caspase-1 and caspase-11 (equivalent to caspase-4/5 in human) are key executioner molecules in cytosolic innate immune responses by triggering pyroptosis, a type of inflammatory necrotic cell death. Caspase-1 is activated by the inflammasome complex whose assembly is mediated a pattern recognition receptor (PRR) and an adaptor protein called ASC upon PRR sensing of pathogen-derived product. Caspase-4/5/11 are activated by direct binding to cytosolic LPS (endotoxin) from bacteria. Activated caspase-1 and caspase-4/5/11 cleave the GSDMD protein to release its N-terminal domain that forms pores on the plasma membrane to trigger pyroptotic cell death. Active caspase-1 can also process pro-IL-1b to promote its secretion, further stimulating inflammation. Pyrin is encoded by the geneMEFV whose genetic mutation causes familial Mediterranean fever (FMF), an autoinflammatory disease in human. A previous study from the Shao laboratory (Xu et al., Nature 2014) discovers that the physiological function of Pyrin is to sense inactivating modification of host Rho GTPases by various bacterial toxins; Pyrin then forms a new inflammasome complex with ASCr, playing an important role in anti-bacterial immunity. Pyrin inflammasome-mediated innate immunity is unique in that it senses bacterial virulence rather than directly recognizing microbial molecules, but its mechanism of activation is unknown.
In the new study, Dr. Feng Shao and his team first found that Pyrin specifically binds to the 14-3-3 family of phospho-serine binding proteins in resting dendritic cells and bone marrow macrophages, which keeps Pyrin in the inactive state. Through extensive mass spectrometry and site-directed mutagenesis analyses, the authors identified Ser-205 and Ser-241 in Pyrin that are phosphorylated; the phosphorylation mediates Pyrin binding to 14-3-3 proteins. Upon toxin stimulation and the resulting modification/inactivation of cellular Rho GTPases, which leads to disruption of actin cytoskeleton, Ser-205 and Ser-241 in Pyrin undergo rapid dephosphorylation. This then causes dissociation of the Pyrin-14-3-3 complex. All these evens show a strong correlation with Pyrin inflammasome activation, suggesting that site-specific dephosphorylation of Pyrin is crucial for its activation in response to toxin stimulation. Moreover, the study also reveals that microtubule drugs, including colchicine commonly used to treat FMF, can effectively block Pyrin inflammasome activation. These drugs do not affect Pyrin dephosphorylation and 14-3-3 dissociation but inhibit Pyrin-mediated ASC aggregation.
The study furthers our understanding of how Pyrin inflammasome senses bacterial toxin modification of host Rho GTPases and provides new insights into the model of cytosolic innate immunity. The study also records an important role of microtubule structure dynamics for Pyrin inflammasome activation and reveals a possible pharmacological mechanism for colchicine treatment of the FMF in clinics.
PhD student Wenqing Gao from the China Agriculture University is the first author of the paper reporting above findings. Other contributors of the study include postdoc fellow Jieling Yang and PhD students Wang Liu and Yupeng Wang from the Shao laboratory. Dr. Feng Shao is the co-corresponding author of the paper. The research was supported by China National Science Foundation, the 973 National High-Tech. Projects, the Strategic Priority Research Program of the Chinese Academy of Sciences, the Beijing Municipal Government and Howard Hughes Medical Institute in the States, and carried out at National Institute of Biological Sciences, Beijing.



