Research News
Studies from Dr. Nan Tang's laboratory demonstrate that the activation of Cdc42/F-actin/MAPK/YAP signaling pathway in alveolar stem cells is critical for mechanical tension induced alveolar regeneration
In August 4th issue of Cell Reports, NIBS Nan Tang lab published a short report entitled “MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration”. In this report, they demonstrate the activation of Cdc42/F-actin/MAPK/YAP signaling pathway in alveolar stem cells is critical for mechanical tension induced alveolar regeneration.
The pulmonary alveolar epithelium, which is essential for the gas exchange function of the lungs, is composed of alveolar type I (AT1) and alveolar type II (AT2) epithelial cells. AT1 cells are large squamous cells that form the epithelial component of the thin air-blood barrier. AT2 cells are small, cuboidal cells that synthesize and secret pulmonary surfactant. Recent studies have established that AT2 cells function as alveolar stem cells in adult lungs. In normal conditions, the turnover of alveolar epithelial cells is slow. After lung injury, AT2 cells are able to quickly undergo self-renewal and to subsequently differentiate into AT1 cells. Compared with the extensive knowledge of the lineage relationships of alveolar epithelial cells, the mechanisms that regulate the proliferation and differentiation of AT2 cells during alveolar regeneration remain poorly understood.
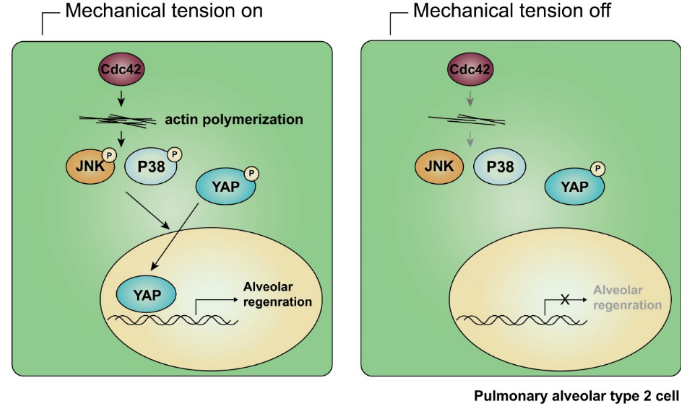
In this study, researchers from Nan Tang’s lab set up a pneumonectomy (PNX) induced mouse lung alveolar regeneration model. After left lung lobe resection, AT2 cells could self-renew and differentiate into AT1 cells to fully restore lung gas exchange function. Previously proposed mechanisms for post-PNX alveolar regeneration include increased mechanical tension, increased pulmonary blood perfusion, and regeneration signals sent from pulmonary endothelial cells. Researchers from Nan Tang’s lab found that increased mechanical tension, which could be indicated by the polymerization of actin, was critical for initialization of alveolar regeneration. They inserted a prosthesis that imitates the shape and size of the left lung to the mouse chest at the time of PNX to block the increased mechanical tension. Prosthesis implanted lungs showed reduced polymerization of actin, decreased AT2 cell proliferation and differentiation, as well as impaired alveolar number and lung volume restoration. These results provide compelling evidence that increased mechanical tension is essential for promoting PNX-induced alveolar regeneration.
In an effort to characterize the genetic programs that regulate mechanical-tension-
induced alveolar regeneration, they used RNA-Seq to identify potential transcriptional regulators that may be involved in regulating gene expression changes in the AT2 cells of PNX mice. They compared genes with up-regulated expression levels in the PNX mice with chromatin immunoprecipitation (ChIP)-sequencing databases, and found that Yes-associated protein (YAP) rank second among six highest-ranked transcription factors. By performing real-time PCR and immunofluorescence staining experiments, they confirmed that the nuclear expression of YAP protein, and the expression levels of previously characterized YAP-target genes, including Birc5, Ctgf, and Cyr61, was increased significantly in the AT2 cells of PNX mice and remained unchanged in the prosthesis mice. Moreover, specifically knockout Yap in AT2 cells leads to impaired alveolar regeneration. Together, these findings establish that YAP function as a mediator of mechanical tension induced signaling in the alveolar epithelium.
MAP kinases have been reported to be activated under mechanical tension. Researchers from Nan Tang’s lab found that p-ERK, p-JNK and p-p38 MAPKs were activated in post-PNX lung tissues but not in prosthesis implanted lung tissues. However, only p-p38 and p-JNK inhibitors but not p-ERK inhibitor could block YAP nuclear expression in the AT2 cells of post-PNX day 5 lung tissues, indicating the essential role of p-JNK and p-p38 but not p-ERK in F-actin-cytoskeleton induced YAP activation.
Cdc42, a small Rho-GTPase, which had been widely reported to be responsible for the regulating of actin polymerization, is known to control the activation of JNK and p38. Researchers from Nan Tang’s lab found that specifically knockout Cdc42 in AT2 cells led to decreased expression levels of p-JNK and p-p38, and weakened actin polymerization in AT2 cells, thus to block the alveolar regeneration after PNX treatment. Based on these experiments, they conclude that Cdc42-mediated F-actin remodeling is critical for activating both JNK and p38 MAPK signaling and YAP in post-PNX alveolar regeneration. It has been shown previously that Cdc42 affects YAP nuclear expression during mouse kidney development. It is tempting to speculate that the Cdc42/F-actin/MAPK/YAP signaling cascade may be a general mechanism for controlling mechanical-tension-induced cellular responses in organ development and tissue repair.
Nan Tang lab’s graduate student Zhe Liu, jointly trained by the National Institutes of Biological Sciences, Peking University, and Tsinghua University, is the first author of this report. Huijuan Wu and Kewu Jiang from Nan Tang’s lab also made very important contributions. Dr. Nan Tang is the corresponding author. This research was supported by the Chinese Ministry of Science and Technology 973 Grants, and was conducted at the National Institute of Biological Sciences, Beijing.



