Research News
Li-Lin Du’s laboratory discovers a new selective autophagy mechanism
Publication Date:2020/08/06
On July 30, 2020, Dr. Li-Lin Du's laboratory published online a paper titled "A UPR-induced soluble ER-phagy receptor acts with VAPs to confer ER stress resistance" in Molecular Cell. This paper reports that ER stress-induced ER-phagy in the fission yeast Schizosaccharomyces pombe requires an autophagy receptor Epr1 and Epr1 confers resistance to ER stress by promoting ER-phagy.
Autophagy is a process by which cytoplasmic materials (cargos) are transported into the lysosome for degradation. In recent years, it has become increasingly recognized that autophagy can be highly selective. The selectivity relies on autophagy receptors, which link specific cargos to the core autophagy-related (Atg) protein Atg8. Environmental and physiological stimuli can cause the accumulation of unfolded proteins in the endoplasmic reticulum (ER), a condition known as ER stress. ER stress triggers the autophagic degradation of the ER (ER-phagy) in various organisms, but the molecular mechanisms, physiological significance, and regulatory factors behind this phenomenon remain largely unknown.
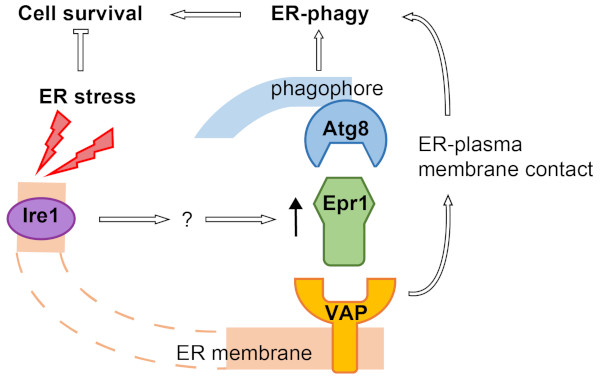
In this study, the authors identified Epr1 as an Atg8-interacting protein. Following the clue that Epr1 localizes to the ER, they found that Epr1 acts as an ER-phagy receptor during ER stress-induced ER-phagy. Unlike previously reported ER-phagy specific receptors, which are all integral ER membrane proteins, Epr1 is a soluble protein. The authors searched for Epr1-interacting proteins and identified two ER transmembrane proteins belonging to the VAP family as Epr1-binding partners. The Epr1-VAP interactions are essential for both the ER localization and the ER-phagy function of Epr1. Biochemical experiments showed that Epr1 can simultaneously interact with Atg8 and VAPs. Genetic analyses demonstrated that the main role of Epr1 in ER-phagy is to bridge an Atg8-VAP association and thereby establish a connection between Atg8 and the ER membrane.
The authors further showed that VAPs contribute to ER-phagy not only by tethering Atg8 to the ER membrane through Epr1, but also by supporting the ER-plasma membrane contact. Moreover, the authors demonstrated that Ire1, a key factor of the unfolded protein response (UPR) signaling pathway, promotes ER stress-induced ER-phagy by upregulating the protein level of Epr1.
Dan Zhao and Chen-Xi Zou are co-first authors of this paper. Dr. Li-Lin Du is the corresponding author. Other contributors include Xiao-Man Liu, Zhao-Di Jiang, Zhong-Qiu Yu, Tong-Yang Du, Fang Suo, Wanzhong He, and Meng-Qiu Dong. This work was supported by the Chinese Ministry of Science and Technology and the Beijing municipal government.
https://www.cell.com/molecular-cell/fulltext/S1097-2765(20)30510-4
Autophagy is a process by which cytoplasmic materials (cargos) are transported into the lysosome for degradation. In recent years, it has become increasingly recognized that autophagy can be highly selective. The selectivity relies on autophagy receptors, which link specific cargos to the core autophagy-related (Atg) protein Atg8. Environmental and physiological stimuli can cause the accumulation of unfolded proteins in the endoplasmic reticulum (ER), a condition known as ER stress. ER stress triggers the autophagic degradation of the ER (ER-phagy) in various organisms, but the molecular mechanisms, physiological significance, and regulatory factors behind this phenomenon remain largely unknown.
In this study, the authors identified Epr1 as an Atg8-interacting protein. Following the clue that Epr1 localizes to the ER, they found that Epr1 acts as an ER-phagy receptor during ER stress-induced ER-phagy. Unlike previously reported ER-phagy specific receptors, which are all integral ER membrane proteins, Epr1 is a soluble protein. The authors searched for Epr1-interacting proteins and identified two ER transmembrane proteins belonging to the VAP family as Epr1-binding partners. The Epr1-VAP interactions are essential for both the ER localization and the ER-phagy function of Epr1. Biochemical experiments showed that Epr1 can simultaneously interact with Atg8 and VAPs. Genetic analyses demonstrated that the main role of Epr1 in ER-phagy is to bridge an Atg8-VAP association and thereby establish a connection between Atg8 and the ER membrane.
The authors further showed that VAPs contribute to ER-phagy not only by tethering Atg8 to the ER membrane through Epr1, but also by supporting the ER-plasma membrane contact. Moreover, the authors demonstrated that Ire1, a key factor of the unfolded protein response (UPR) signaling pathway, promotes ER stress-induced ER-phagy by upregulating the protein level of Epr1.
Dan Zhao and Chen-Xi Zou are co-first authors of this paper. Dr. Li-Lin Du is the corresponding author. Other contributors include Xiao-Man Liu, Zhao-Di Jiang, Zhong-Qiu Yu, Tong-Yang Du, Fang Suo, Wanzhong He, and Meng-Qiu Dong. This work was supported by the Chinese Ministry of Science and Technology and the Beijing municipal government.
https://www.cell.com/molecular-cell/fulltext/S1097-2765(20)30510-4



