Research News
Aug 15, 2018 - Studies from Dr. Feng Shao’s laboratory reveal that ADP-heptose, a precursor sugar molecule in bacterial LPS biosynthesis is recognized by a novel immune receptor kinase ALPK1, which stimulates host inflammatory responses.
Aug 15, 2018 - Studies from Dr. Feng Shao’s laboratory reveal that ADP-heptose, a precursor sugar molecule in bacterial LPS biosynthesis is recognized by a novel immune receptor kinase ALPK1, which stimulates host inflammatory responses.
Central to innate immunity is the pattern recognition receptor (PRR) that senses various pathogen-associated molecular patterns (PAMPs). The membrane-bound Toll-like receptors are the prototypical PRRs. In the 1990s, Bruce Beutler, Ruslan Medzhitov and Charles Janeway et al. identity TLR4 as the receptor for bacterial lipopolysaccharide (LPS, also known as endotoxin) and stimulates NF-κB-mediated expression of cytokines and proinflammatory factors.
Dr. Feng Shao’s laboratory has been focusing on the molecular mechanism underlying cytosolic sensing of bacteria. In the last few years, they have identified several bacterial PRRs. These include 1) the NAIP family that serves as cytosolic receptors for bacterial flagellin as well as components of the type III secretion system (T3SS) (Zhao et al., Nature 2011), 2) Pyrin, encoded by the familial Mediterranean fever disease gene, that senses the inactivating modifications of host Rho GTPases by pathogenic bacteria or toxins (Xu et al., Nature 2014), 3) caspase-11/4/5 that act as the cytosolic receptors for bacterial LPS and are activated by binding to the lipid A moiety of LPS. Activation of these bacterial PRRs lead to inflammasome complex assembly, which induces activation of the pore-forming protein GSDMD and pyroptotic cell death (Shi et al., Nature, 2015; Ding et al., Nature 2016). In the new work, they further discover that a cytosolic kinase ALPK1 (alpha-kinase 1) directly recognizes ADP-heptose, a bacterial sugar metabolite in the LPS biosynthesis pathway, and this recognition stimulates the pro-inflammatory NF-κB signaling and innate immune responses. The work entitled “Alpha-kinase 1 is a cytosolic innate immune receptor for bacterial ADP-heptose” is published in the journal Nature as Advance Online Publication on Aug. 15, 2018.
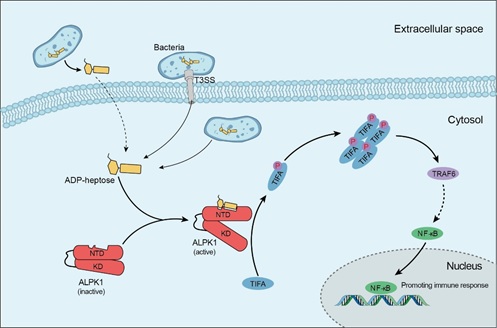
Previous studies have shown that many Gram-negative bacterial pathogens such as Yersinia and enteropathogenic E. coli. can activate host NF-κB proinflammatory signaling in the T3SS-dependent manner, but the underlying mechanism is unknown. In this study, researchers from the Shao laboratory employed transposon screen in Y. pseudotuberculosis, combined with biochemical analyses, discovered that ADP-heptose, a precursor sugar molecule involved in bacterial LPS biosynthesis, determines T3SS-dependent NF-κB activation and induction of the inflammatory responses. ADP-heptose is present in all Gram-negative and certain Gram-positive bacteria. They further discovered that addition of ADP-heptose to host cells without transfection is sufficient to induce NF-κB activation. To understand host sensing mechanism for ADP-heptose, the researchers performed FACS-based genome-wide CRISPR-Cas9 screen in host cells, and uncovered the ALPK1-TIFA-TRAF6 pathway. ALPK1 is a cytosolic kinase, consisting of an N-terminal domain (NTD) and a C-terminal kinase domain (KD) linked by a long extended unstructured region. Through a series of biochemical and cell biological experiments, they found that the NTD and the KD have constitutive interactions; the NTD can directly bind ADP-heptose, which, through conformational changes, activates the KD to phosphorylate the TIFA. The phosphorylated TIFA then oligomerizes to bind and activate TRAF6 for downstream NF-κB activation. They further determined crystal structure of ALPK1-NTD in complex with ADP-heptose, which provides atomic details on how the ALPK1 receptor recognizes the sugar ligand.
A recent study proposes that heptose 1,7-bisphosphate (HBP), the precursor of ADP-heptose in LPS biosynthesis, can induce TIFA-dependent NF-κB activation when transfected into host cells. The Shao team further found that HBP can not enter host cells and is deficient in stimulating ALPK1 activation; however, HBP can be metabolized by host adenylyltransferases into ALPK1-competent ADP-heptose 7-P despite a much lower activity than ADP-heptose.
The researchers also found that injection of ADP-heptose but not HBP into mice could induce strong inflammation. They then generated Alpk1-deficient mice, which show no inflammation following ADP-heptose injection. When mice were infected with a lung pathogen Burkholderia cenocepacia, decreased lung inflammation and a higher number of bacteria are observed in the Alpk1-deficient mice compared with wild-type mice. These suggest that ALPK1 sensing of ADP-heptose plays an important role in innate immune defenses against bacterial infection. The study identifies a novel cytosolic receptor kinase ALPK1 that recognizes a bacterial sugar metabolite ADP-heptose, representing a conserved and generic form of innate sensing of bacteria. The findings enrich and change our understanding of LPS sensing and LPS-induced inflammation. The cell entry property of ADP-heptose also suggests that this sugar molecule has the potential to be developed as an effective immune modulator or vaccine adjuvant.
Ping Zhou, a postdoc fellow in the Shao laboratory and Yang She, a guest PhD student from Institute of Biophysics (CAS), are co-first authors of the paper. Other contributors include Dr. Na Dong (now at Chinese Agriculture University), Dr. Peng Li, Huabin He, Yue Xu, Dr. Wenqing Gao, Dr. Jingjin Ding in the Shao laboratory, Qingcui Wu (NIBS Chemical Center), Shan Lu, Yong Cao, Dr. Mengqiu Dong, Dr. Da-Cheng Wang, Dr. Alla Zamyatina and her student Alessio Bori (University of Natural Resources and Life Sciences, Vienna, Austria) as well as Xiaojun Ding at the Beijing Mingde Zhengkang Technologies Co., Ltd. Dr. Feng Shao is the corresponding author of the study. The research was supported by the Basic Science Center of the National Science Foundation, the National Key Research and Development Project, the Strategic Priority Research Program of the Chinese Academy of Science, as well as a grant from the Austrian Science Fund, and carried out at National Institute of Biological Sciences, Beijing.