Research News
Dr. Xin-Jian He’s lab reported the identification of a set of protein complexes involved in heterochromatin silencing in Arabidopsis.
Aug 13, 2018 ---- Dr. Xin-Jian He's lab published an article titled “The PEAT protein complexes are required for histone deacetylation and heterochromatin silencing” online in EMBO Journal. This paper reported the identification of a set of protein complexes involved in histone deacetylation, Pol IV-dependent siRNA production, heterochromatin silencing in Arabidopsis.
In eukaryotes, heterochromatin regions are typically subjected to transcriptional silencing. DNA methylation has an important role in such silencing and has been studied extensively. However, little is known about how methylated heterochromatin regions are subjected to silencing. We conducted a genetic screen and identified an epcr (enhancer of polycomb‐related) mutant that releases heterochromatin silencing in Arabidopsis thaliana. We demonstrated that EPCR1 functions redundantly with its paralog EPCR2 and interacts with PWWP domain‐containing proteins (PWWPs), AT‐rich interaction domain‐containing proteins (ARIDs), and telomere repeat binding proteins (TRBs), thus forming multiple functionally redundant protein complexes named PEAT (PWWPs‐EPCRs‐ARIDs‐TRBs) (Figure 1). The PEAT complexes mediate histone deacetylation and heterochromatin condensation and thereby facilitate heterochromatin silencing. In heterochromatin regions, the production of small interfering RNAs (siRNAs) and DNA methylation is repressed by the PEAT complexes (Figure 2). The study reveals how histone deacetylation, heterochromatin condensation, siRNA production, and DNA methylation interplay with each other and thereby maintain heterochromatin silencing.
Lian-Mei Tan and Cui-Jun Zhang from the He lab are the co-first authors of the paper. Dr. Xin-Jian He from National Institute of Biological Sciences is the corresponding author. The study was supported by the Chinese Ministry of Science and Beijing Municipal Government.
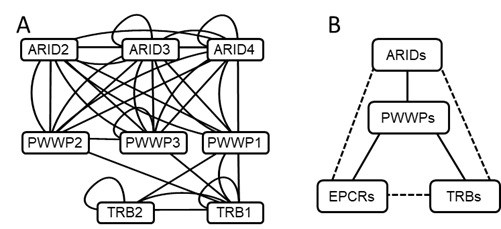
Figure 1. EPCR1/2 interact with ARID2/3/4, PWWP1/2/3, and TRB1/2, and form complexes.
(A) The diagram of protein-protein interactions that were detected in yeast two-hybrid assays. (B) The diagram indicates the interactions among EPCR1, PWWP2, ARID2, and TRB1 as determined by pull-down assay, yeast two-hybrid, and co-IP. The solid line and the broken line indicate, respectively, direct and indirect protein-protein interactions.
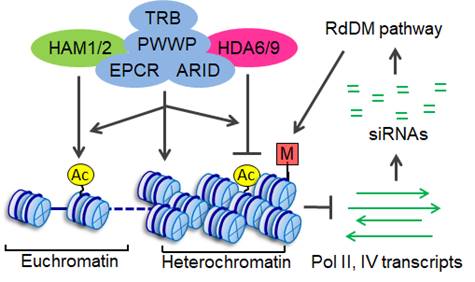
Figure 2. Model for the role of the PEAT complexes in chromatin regulation.



