Research News
Studies from Dr. Feng Shao’s laboratory reveal that bacterial virulence effector IcsB from Shigella flexneri employs a novel lysine fatty acyltransferase activity to modify multiple host proteins to counteract host defenses and promote pathogenesis.
July 30, 2018 - Studies from Dr. Feng Shao’s laboratory reveal that bacterial virulence effector IcsB from Shigella flexneri employs a novel lysine fatty acyltransferase activity to modify multiple host proteins to counteract host defenses and promote pathogenesis. The work entitled “Nε-fatty acylation of multiple membrane-associated proteins by Shigella IcsB effector to modulate host function” is published in the journal Nature Microbiology.
Shigella flexneri is the major cause for bacillary dysentery and Shigellosis. Like many other Gram-negative bacterial pathogens, S. flexneri uses a type III secretion system (T3SS) to inject multiple effector proteins into host cells to interfere with or block host defenses and promote infection. IcsB is one of the earlies identified T3SS effectors from S. flexneri and has been known to be critical for Shigella pathogenesis. Previous studies show that IcsB can modulate multiple host cellular processes including promoting intercellular spread through lysis of bacterial protrusion, facilitating bacterial escape from the Septin cage, and evading antibacterial autophagy. However, the mechanism underlying these functions of IcsB has been unknow.
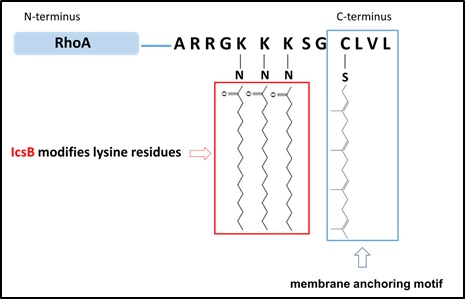
In this study, researchers from Dr. Feng Shao’s laboratory first discovered that ectopic expression of IcsB shows a sever cytotoxicity of inhibiting the growth of yeast and mammalian cells. Cells expressing IcsB undergo evident morphological changes of rounding up and detaching from the culture dish. This indicates that a direct or indirect effect of disrupting the cytoskeleton structure by IcsB. Further studies from the researchers show that IcsB can disrupt the actin filamentous fibers in host cells by inactivating the Rho family small GTPases that are known to act as a switch in controlling actin dynamics. Through a series of rigorous biochemical and cell biological experiments, they revealed that IcsB harbors a long-chain fatty acyltransferase (C18) activity and modifies lysine residues in the C-terminal polybasic region (PBR) of Rho GTPases. This novel posttranslational modification generates an inactive Rho that lost the ability of regulating the actin cytoskeleton structure. This finding explains the growth-inhibition cytotoxicity of IcsB in yeast and mammalian cells.
In above studies, the researchers noticed that 1) IcsB recognition of Rho does not require a specific sequence motif but only the PBR preceding the lipidated CAAX motif that is commonly present in many eukaryotic proteins; 2) Modification of Rho can not explain the pleiotropic functions of IcsB, particularly inhibition of the host autophagy pathway. With these questions in mind, the researchers designed an ingenious chemical proteomics experiment, which can capture all the long-chain fatty acylated proteins induced by IcsB from S. flexneri-infected cells. These IcsB-modified proteins are then identified by isotope-based quantitative mass spectrometry. Using this approach, the researchers found that IcsB can modify more than 60 different host proteins in S. flexneri-infected cells. These include the Rho family of GTPases already identified, the Ras and Rab families of small GTPases, as well as the SNARE and Septin families of non-small GTPases proteins. All these proteins share the PBR motif and regulate multiple membrane trafficking-related processes in the host cell. After screening all these IcsB substrates, the researchers found that IcsB efficiently modifies the CHIMP5 protein to block the antibacterial autophagy in the host.
The study for the first time reveals a novel lysine fatty acyltransferase activity in Shigella T3SS effector IcsB. This activity can modify Rho GTPases to disrupt actin cytoskeleton structure in host cells. IcsB can also modify a large group of host membrane or membrane-associated proteins including other members of the Ras superfamily of small GTPases and other non-small GTPase proteins, which explains its pleiotropic functions of modulating multiple host cellular processes in promoting S. flexneri survival and replication in the host.
Wang Liu (a PhD student of the PTN program) and Yan Zhou (a former PhD student from Beijing Normal University, currently working at Zhejiang University) in the Shao laboratory, and Tao Peng (a former postdoc from Dr. Howard C. Hang’s laboratory at Rockefeller University, currently at Peking University Shenzhen Graduate School) are co-first authors of the study. Other contributors include Drs. Ping Zhou and Zilin Li, Haoyu Zhong, and Yue Xu from the Shao laboratory, Xiaojun Ding and Dr. She Chen from NIBS proteomic facility. Drs. Howard C. Hang and Feng Shao are co-corresponding authors. The research was supported by the Basic Science Center of the National Science Foundation, the National Key Research and Development Project, the Beijing Scholar Program of Beijing Municipal Government, the Strategic Priority Research Program of the Chinese Academy of Science, as well as a grant from National Institute of Health (US), and carried out at National Institute of Biological Sciences, Beijing and the Rockefeller University.



