Research News
Studies from Dr. Tao Wang’s laboratory discover that V-ATPase is required for membrane protein tracfficking in Drosophila visual system
On July 1, 2018-Studies from Dr. Tao Wang's laboratory revealed that the V1 component of V-ATPase play key roles in post-Golgi trafficking of Rh1. The work entitled “The V-ATPase V1 subunit A1 is required for rhodopsin anterograde trafficking in Drosophila” is published online as a cover article in the journal Molecular biology of the cell (10.1091/mbc.E17-09-0546).
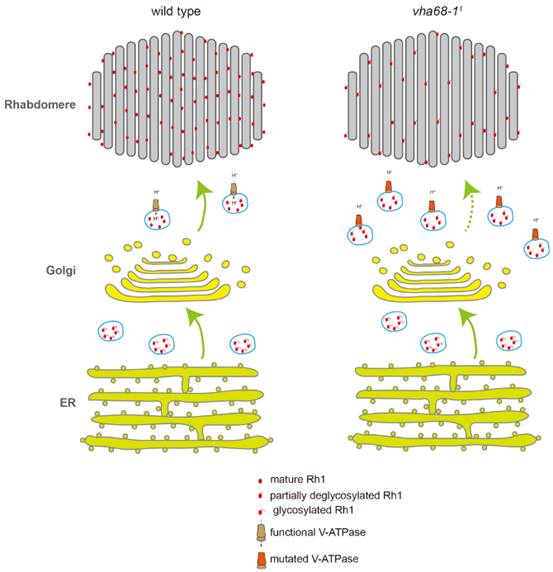
The major rhodopsin in Drosophila, Rh1, is synthesized in the endoplasmic reticulum (ER), where it undergoes N-linked glycosylation and strict quality control before being trafficked to the rhabdomeres. Proper targeting of Rh1 to the rhabdomere is crucial for normal phototransduction and photoreceptor cell survival. A wide array of chaperones within the ER ensure proper biosynthesis and folding of rhodopsin. After Rh1 exits the ER, it is subjected to additional quality control, including a series of deglycosylation events, which are essential for the transport and function of newly synthesized rhodopsin. Rab11, which is present in the trans-Golgi network and post-Golgi vesicles, is required for trafficking from the Golgi to the apical rhabdomere membrane. However, other than the role played by Rab11, very little is known about how anterograde transport of rhodopsin from Golgi to the rhabdomere is regulated.
In the present study, the authors performed ethyl methanesulfonate (EMS) mutagenesis in Drosophila and screened chromosome 2L for mutations that affect rhodopsin homeostasis and photoreceptor cell integrity with in vivo fluorescent imaging of Rh1-GFP. A mutation in vha68-1, which encodes the V-ATPase catalytic subunit A (isoform 1) of the V1 subcomplex was isolated.
The authors found that anterograde trafficking of membrane proteins, including Rh1 and TRP, was defective in vha68-11 photoreceptor cells. Moreover, in vha68-11 flies, Rh1 largely colocalized with Rab11, which is a marker for post-Golgi secretory pathway. The authors performed a pulse-chase assay to track newly synthesized Rh1 in the vha68-11 mutant flies. The results indicated that Rh1 anterograde trafficking is slow in vha68-11 photoreceptors. As V-ATPase is crucial for maintain pH in the cell, the authors measured pH in the photoreceptor cell using pH sensitive GFP variant called pHluorin. The pH was increased in the vha68-11 flies compared with wild type flies. In addition, the authors found that the deglycosylation process was blocked after Golgi step in the vha68-11 flies. In summary, the authors propose a model for the role of V-ATPase in the membrane protein tracfficking process. In wild-type flies, Rh1 is synthesized in the ER and undergoes N-glycosylation. Oligosaccharides are sequentially removed in the Golgi and secretory vesicles before mature Rh1 is delivered to the rhabdomere. In vha68-11 mutant flies, post-Golgi secretory vesicles fail to maintain low pH level because of V-ATPase malfunction. As a result, Rh1 is not fully deglycosylated, accumulating in secretory vesicles within the photoreceptor cell body. Levels of functional Rh1 are therefore reduced in the rhabdomere. This study also provide an important animal model system for diseases associated with V-ATPase dysfunction.
Haifang zhao, a PhD student of the PTN program from Dr. Tao Wang’s lab is the first author of this article. Other contributor includes Jing Wang from the Tao Wang’s laboratory. Dr. Tao Wang is the corresponding author. The study was supported by the Chinese Ministry of Science and Technology 973 Grants, and was conducted at the National Institute of Biological Sciences, Beijing.