Research News
Dr. Nan Tang’s laboratory revealed the mechanism that controls pulmonary alveolar development
On 2/05/2018, Dr. Nan Tang’s laboratory published a paper entitled “The strength of mechanical forces determines the differentiation of alveolar epithelial cells” in Developmental Cell. In this study, they demonstrated that mechanical forces and local growth factors synergistically control pulmonary alveolar epithelial cell differentiation.
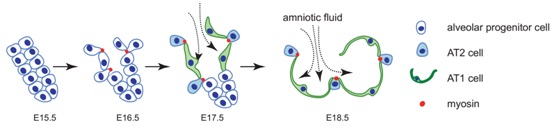
The differentiation of pulmonary alveolar epithelial type I (AT1) and type II (AT2) cells is essential for the lung gas exchange function. Disruption of this process results in neonatal death or in severe lung diseases that last into adulthood. Despite the vital importance of the proper differentiation of alveolar epithelial cells, very little is known about the mechanism that controls alveolar epithelial cell fate specification.
At late embryonic stages, the distal airway tubules begin to dilate to form thin-walled distal sacs and progenitor cells differentiation into AT1 and AT2 cells. Considering both the rapidity of distal sac formation and the complex biophysical and biochemical environment of the developing lung, an ability to monitor cellular processes in real time could provide important insights about the mechanisms that control this highly dynamic developmental process. Researchers established bothin vivo and ex vivo embryonic lung live imaging systems, and used them to continuously monitor the cellular processes of alveolar progenitor cells during alveolar epithelial cell differentiation. Researchers found that a large subset of alveolar progenitor cells is able to protrude from the airway epithelium toward the mesenchyme in an FGF10/FGFR2 signaling-dependent manner. During late embryonic development,the fetal breath movements become stronger and inhaled amniotic fluids reach to the distal airways. Mechanical forces generated by the inhalation of amniotic fluids promote the non-protruded cells become flattened and differentiate to AT1 cells. While the cell protrusion process results in reduced apical surface area and accumulation of apical myosin in protruded cells, and prevents them from being flattened by mechanical force, thereby ensuring their AT2 cell fate. This study demonstrated a sophisticated developmental process in which cell differentiation is coordinated by both mechanical forces and local growth factor.
Jiao Li, a PhD student of China Argriculture University from Dr. Nan Tang’s lab, is first authors of this article. Other contributors include Zheng Wang, Kewu Jiang, Qiqi Chu, and Juan Li from Dr. Nan Tang’s lab. Dr. Nan Tang is the corresponding author. This research was supported by the National key research and development program of China, and carried out at National Institute of Biological Sciences, Beijing.



