Research News
Dec. 12, 2016--Studies from Dr. Feng Shao’s laboratory identify a novel phosphatidylinositide 4-kinase activity in bacterial pathogens. The work entitled “Modulation of membrane phosphoinositide dynamics by the phosphatidylinositide 4-kinase activity of t
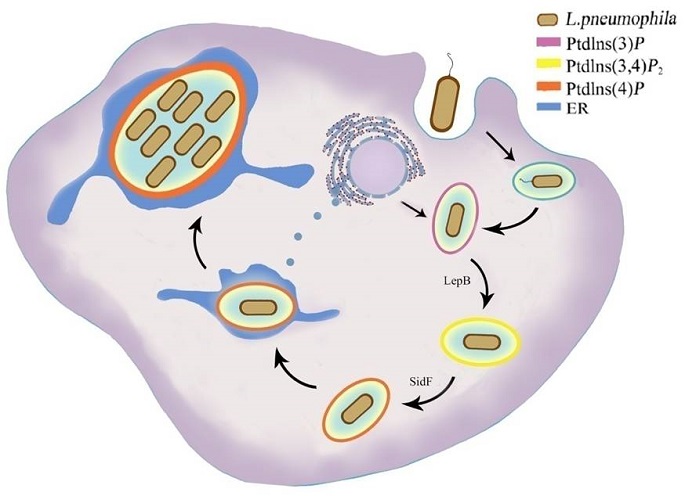
In the new study, Dr. Shao and his team first found that the N-terminal region of LepB contains a cryptic independent functional domain (LepB_NTD) that can kill yeast cells. Interestingly, crystal structure of LepB suggests that LepB_NTD is similar to some atypical kinases, suggesting a putative kinase activity. They also found that ectopic expression of LepB_NTD in eukaryotic cells could disrupt the trans- and middle-Golgi structure without affecting the cis-Golgi, which requires LepB_NTD kinase activity according to structure-based mutagenesis analyses. After failing to identify any protein substrate of LepB_NTD kinase activity, the researchers noticed that the cellular effect of LepB_NTD resembles that of overexpression of the PtdIns4P-binding PH domain reported in the literatures. Using a panel of fluorescent phosphoinositide probes, they found that LepB_NTD expression could cause disappearance of PtdIns3P on the inner membrane and alter the distribution of PtdIns(3,4)P2. The researchers then hypothesize that LepB_NTD may work on a phosphoinositide as its substrate. Results of in vitro kinase assays performed with a panel of phosphoinositides demonstrate that LepB_NTD has a robust phosphoinositide kinase activity that specifically converts PtdIns3P into PtdIns(3,4)P2. It is known that the LCV is enriched with PtdIns4P, but how PtdIns4P is synthesized has been unknown. The shao team further discovered that LepB_NTD works together with another L. pneumophila type IV effector SidF that is known to possess phosphoinositide phosphatase activity of converting PtdIns(3,4)P2 into PtdIns4P to generate PtdIns4P on the LCV membrane.
This study for the first time identifies a phosphatidylinositide kinase activity in bacterial pathogens, which differs from all known eukaryotic phosphatidylinositide kinases and adds a phosphate on the D4 position of the phosphoinositol ring. LepB_NTD can serve as a useful tool for studying phosphoinositide dynamics and membrane transport. The study also suggests that that there are other yet to be identified bacterial phosphoinositide kinase effectors that play important functions in bacterial manipulation of membrane trafficking and promoting bacterial replication inside host cells.
Postdoc fellow Na Dong and PhD student Miao Niu are co-first authors of the paper reporting above findings. Other contributors of the study include Liyan Hu, Dr. Qing Yao and Rui Zhou of the Shao laboratory. Drs. Na Dong and Feng Shao are co-corresponding authors of the paper. The research was supported by China National Science Foundation, the 973 National High-Tech. Projects, the Strategic Priority Research Program of the Chinese Academy of Sciences, the Beijing Municipal Government and Howard Hughes Medical Institute in the States, and carried out at National Institute of Biological Sciences, Beijing.