Research News
Microenvironmental regulation of stem cell differentiation by PcG and TrxG genes.
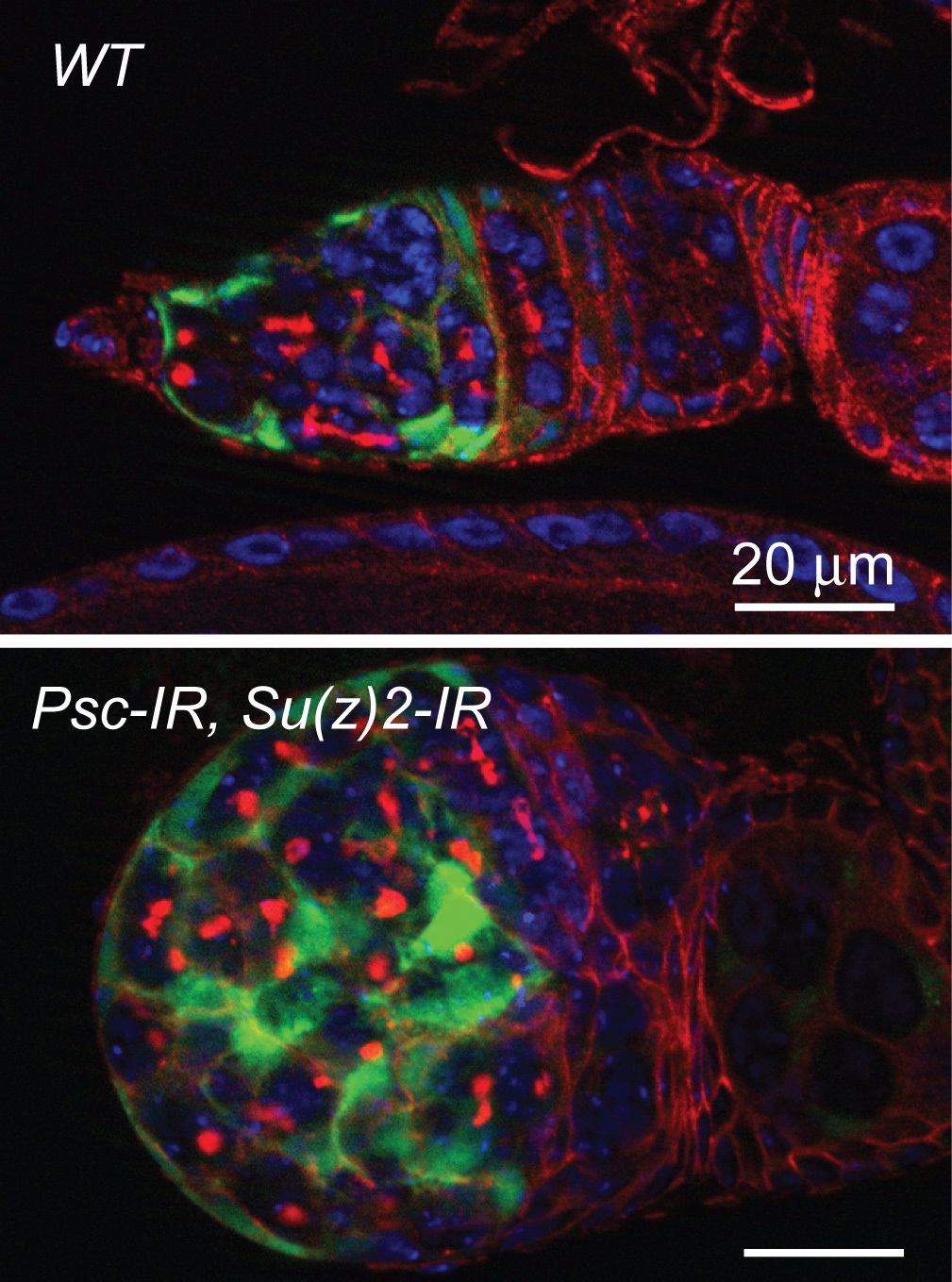
In Oct. 1st issue of Development, NIBS Xi lab published a featured article entitled “Control of germline stem cell differentiation by Polycomb and Trithorax group genes in the niche microenvironment”. In this article, the authors describe a novel role for PcG and TrxG genes in the niche microenvironment in controlling germline stem cell differentiation in Drosophila.
Adult stem cell is important for tissue homeostasis due to its two characteristics: self-renew and differentiation. These stem cells commonly reside in a specialized microenvironment, named niche, where they receive self-renew signal to keep the undifferentiated state. In adult Drosophila ovary, two or three GSCs reside at the anterior tip of germarium where they contact the supporting niche composed of cap cells and terminal filament cells. The niche produces a Bone morphogenetic protein (BMP) ligand Dpp, which directly actives BMP signaling pathway in GSCs to suppress the expression of differentiation-promoting gene bam. So it is important to restrict dpp expression in niche cells in a reasonable level, which ensures the balance of GSC self-renew and differentiation. During embryogenesis, the repressive Polycomb group (PcG) complexes and active Trithorax group (TrxG) complexes play antagonistic roles in regulating Hox genes to form stereotyped body pattern. In this study, the authors report that disruption of the PRC1, specifically in the supporting escort cells, causes blockage of cystoblast differentiation and germline stem cell- like tumor formation. BMP signaling activity is usually restricted to GSCs and quickly declined in daughter cystoblasts. However, in the germaria with escort cell-specific depletion of PRC1, the expression of BMP signaling reporters, pMad and Dad-lacZ, were expanded to the spectrosome-containing cells outside the cap cell niche. In the meanwhile, the expression of differentiation-promoting gene bam was reduced, as the bam-GFP was undetectable in virtually all spectrosome-containing cells. The increased BMP signaling is due to aberrant expression of dpp in escort cells especially the dpp-RB transcript. Knocking down dpp could suppress the GSC-like tumor phenotype. The authors further demonstrate that depletion of PRC1 does not transform escort cells into cap cells or pre-follicle cells. Interestingly, activation of dpp in escort cells requires the function of the TrxG gene brahma (brm), suggesting that loss of PRC1 in escort cells causes Brm-dependent dpp expression. This is the first demonstration of a requirement for balanced activity between PcG and TrxG genes in an adult stem cell niche, and disruption of this balance could lead to the loss of tissue homeostasis and tumorigenesis.
Xuewen Li, a graduate student jointly trained by NIBS and China Agriculture University, and Postdoc Fu Yang at NIBS, are co-first authors of this article. Other contributors include Hongyan Chen, Bowen Deng and Xinghua Li from NIBS. Dr. Rongwen Xi is the corresponding author. The study was supported by the Chinese Ministry of Science and Technology 973 Grants.